How We Clean Carpets: The Chemistry of Cleaning
Published on Jan 2, 2020

If you’ve ever wondered why certain cleaning products are better at certain jobs around the home than others, then this article is for you.
We’re taking you back to high school science class for this week’s blog and sharing a little knowledge about the chemistry involved in cleaning.
Chemistry, more specifically the pH Scale, is a bigger part of your everyday life than you may think.
For our Electrodry technicians, a solid understanding of the pH scale is crucial and it can be the difference between a good job and a great one for our customers.
It plays a key role in how effectively you’re cleaning your house.
The pH Scale
pH stands for ‘potential of hydrogen’ and the scale measures how many hydrogen ions are in a substance. Where that substance sits on the scale determines whether it is acidic, neutral or alkaline.
Here’s a great image of the pH scale.
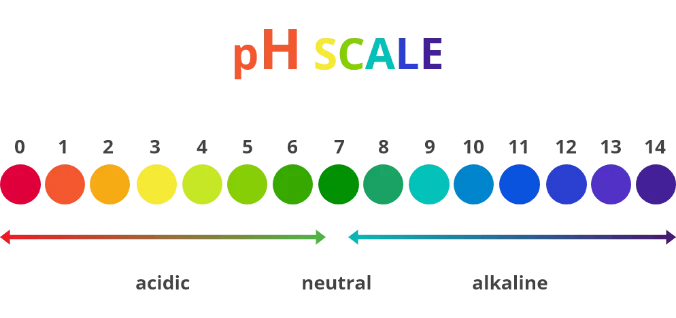
There are 14 points on the scale, and anything with a low concentration of hydrogen ions is acidic (rates 0-6) and a substance with a high concentration of hydrogen ions (8-14) is alkaline. A substance that rates a 7 on the scale is neutral.
The pH Scale in Everyday Life

It’s likely you have already used a number of substances throughout your day so far that sit on the scale somewhere. Any water-based substance has a pH rating and includes many foods, drinks, beauty items and yes, cleaning products.
To give you a bit of an idea of what is classified as acidic and alkaline in our day-to-day lives, we’ve created this graphic.
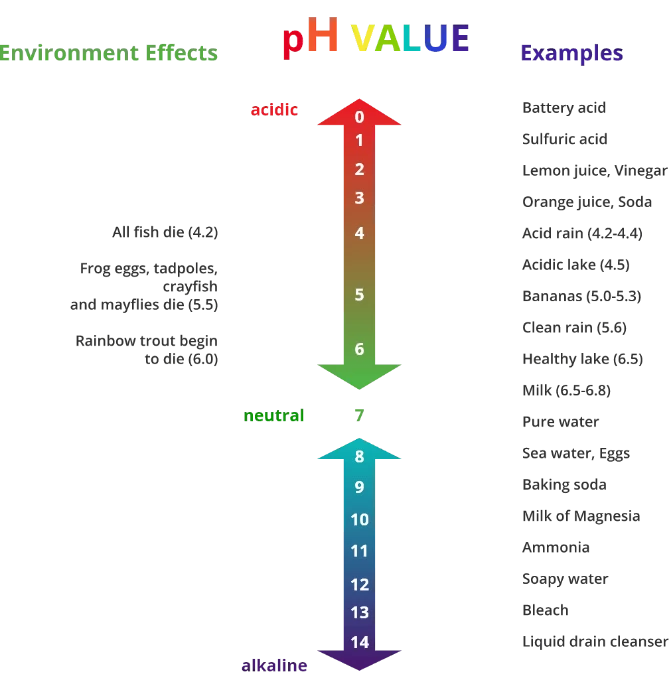
The pH Scale of Cleaning Chemicals

Despite popular belief, strongly alkaline substances are not necessarily better cleaners. The goal of cleaning is to neutralise the stain’s pH level, so it all depends on the acidity or alkalinity of the soiled area you’re trying to clean.
Put simply, acid neutralises alkaline, and alkaline neutralises acid. Here is what you need to know:
An alkaline cleaner generally contains potent bases including potassium hydroxide and sodium hydroxide. It dissolves acidic substances like fats, oils and grease (therefore includes many foods). Think bleach, oven cleaners and multi-purpose sprays such as Ajax.
Acidic cleaners are great at dissolving alkaline substances like mineral deposits or rust, making them great for cleaning many areas of your bathroom. Most toilet and tile cleaners have an acidic pH level.
The diagram below demonstrates where some cleaning products sit on the scale.
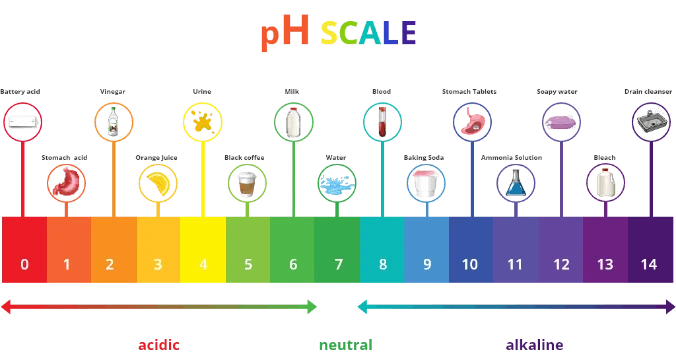
Note: Extremely alkaline (drain cleaner and bleach) or acidic (battery acid) substances are reactive, meaning that they can cause burns.
How does Electrodry use the pH scale in cleaning?

So, how do we do it? As we get your carpet clean, we restore the pH balance, so we adhere to the acidic cleaner for alkaline stains (and vice-versa) rule too.
We also ensure that when high pH (Alkaline) cleaners have been used to treat fatty, oily and greasy stains, we use sufficient levels of a low pH (acidic) neutraliser to re-balance the carpet’s chemical balance.
Getting the right chemical balance allows our technicians to bring back the natural brightness for carpets and fabrics that were manufactured with acid dyes (most fabrics). A chemically balanced fabric will also be softer, dry more quickly and be less likely to carry a chemical residue that will contribute to re-soiling.
What Your Professional Carpet Cleaner Knows About pH Levels That You Probably Don't

-
Using an extremely high or low pH substance can damage surfaces (vinegar can etch marble surfaces while strong alkaline cleaners can instantly and often irreversibly damage carpet dyes).
-
High pH chemicals can unset acidy dyes, leading to discolouration or colour leaching.
-
High pH chemicals can cause chemical burns on wool carpets.
-
When cleaning tiles, acidic cleaning products are required to break down soaps and cleaning product residues, whilst acidic cleaning products treat grease, oil and fat.
-
Fabrics treated with acidic cleaning solutions are less likely to suffer watermarking.
-
Pet urine starts its life as an acid but becomes a strong alkaline once it dries and accordingly needs to be treated with an acidic product.




